 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| IGE-M52H3 | Mouse | Mouse IgE Fc Protein, His Tag (MALS & SPR verified) |  |

|
|
| IGE-C52H3 | Cynomolgus | Cynomolgus IgE Fc Protein, His Tag (MALS & SPR verified) |  |
|
|
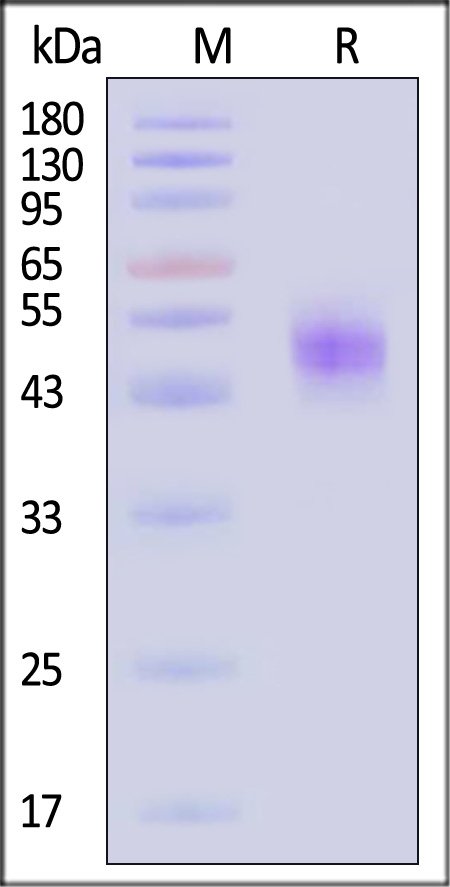
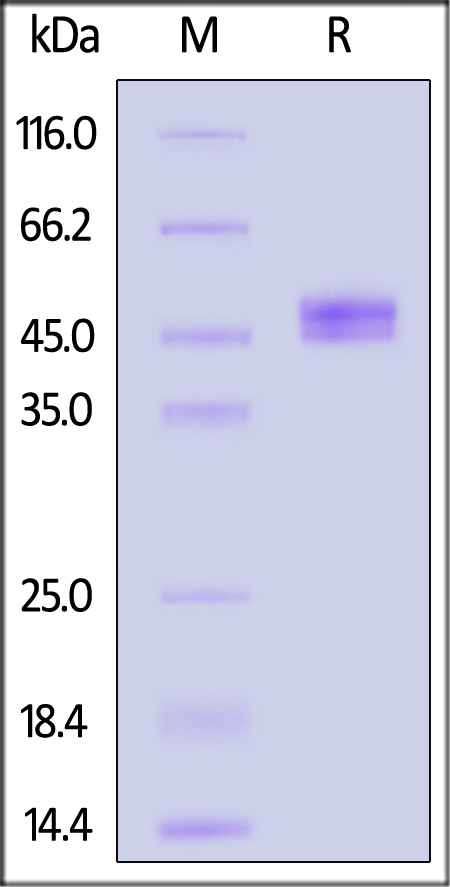
| IGE-H52H9 | Human | Human IgE Fc Protein, His Tag |  |
|
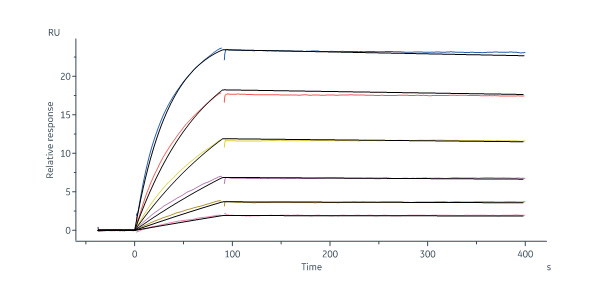
Human Fc epsilon RI alpha, His Tag (Cat. No. FCA-H5228) immobilized on CM5 Chip can bind Mouse IgE Fc Protein, His Tag (Cat. No. IGE-M52H3) with an affinity constant of 0.391 nM as determined in a SPR assay (Biacore 8K) (QC tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Omalizumab biosimilar (IBC Generium) | GNR-044 | Approved | Ibc Generium | Genolair | Russian Federation | Chronic Urticaria; Asthma | Generium Pharmaceuticals | 2022-02-09 | Chronic Urticaria; Asthma | Details |
| Omalizumab biosimilar (Mabtech) | STI-004; CMAB007; CMAB-007 | Approved | Taizhou Maibo Taike Biotechnology Co Ltd | 奥迈舒 | Mainland China | Asthma | Taizhou Mabtech Pharmaceutical Co Ltd | 2023-05-19 | Chronic Urticaria; Allergic asthma; Asthma | Details |
| Omalizumab | GN-1560; RG-3648; IGE-025A; rhuMAb-E25; R-3648; E-25; IGE-025 | Approved | Novartis Pharma Ag, Genentech Inc | Xolair, 茁乐 | Australia | Asthma; Chronic Urticaria | null | 2002-01-01 | Pulmonary Disease, Chronic Obstructive; Job Syndrome; Asthma; Sinusitis; Urticaria; Esophagitis; Pemphigoid, Bullous; Bronchitis; Peanut Hypersensitivity; Allergic asthma; Infertility, Female; Angioedema; Dermatitis, Atopic; Mastocytosis; Hypersensitivity; Rhinitis; Gastroenteritis; Respiratory Tract Infections; Eosinophilic Esophagitis; Lupus Erythematosus, Systemic; Food Hypersensitivity; Immune System Diseases; Nose Diseases; Coronavirus Disease 2019 (COVID-19); Milk Hypersensitivity; Colonic Neoplasms; Sjogren's Syndrome; Nasal Polyps; Chronic Urticaria; Autism Spectrum Disorder; Rhinitis, Allergic, Seasonal; Anaphylaxis; Drug Hypersensitivity | Details |
| Omalizumab biosimilar (IBC Generium) | GNR-044 | Approved | Ibc Generium | Genolair | Russian Federation | Chronic Urticaria; Asthma | Generium Pharmaceuticals | 2022-02-09 | Chronic Urticaria; Asthma | Details |
| Omalizumab biosimilar (Mabtech) | STI-004; CMAB007; CMAB-007 | Approved | Taizhou Maibo Taike Biotechnology Co Ltd | 奥迈舒 | Mainland China | Asthma | Taizhou Mabtech Pharmaceutical Co Ltd | 2023-05-19 | Chronic Urticaria; Allergic asthma; Asthma | Details |
| Omalizumab | GN-1560; RG-3648; IGE-025A; rhuMAb-E25; R-3648; E-25; IGE-025 | Approved | Novartis Pharma Ag, Genentech Inc | Xolair, 茁乐 | Australia | Asthma; Chronic Urticaria | null | 2002-01-01 | Pulmonary Disease, Chronic Obstructive; Job Syndrome; Asthma; Sinusitis; Urticaria; Esophagitis; Pemphigoid, Bullous; Bronchitis; Peanut Hypersensitivity; Allergic asthma; Infertility, Female; Angioedema; Dermatitis, Atopic; Mastocytosis; Hypersensitivity; Rhinitis; Gastroenteritis; Respiratory Tract Infections; Eosinophilic Esophagitis; Lupus Erythematosus, Systemic; Food Hypersensitivity; Immune System Diseases; Nose Diseases; Coronavirus Disease 2019 (COVID-19); Milk Hypersensitivity; Colonic Neoplasms; Sjogren's Syndrome; Nasal Polyps; Chronic Urticaria; Autism Spectrum Disorder; Rhinitis, Allergic, Seasonal; Anaphylaxis; Drug Hypersensitivity | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Omalizumab biosimilar (Synermore Biologics) | SYN-008 | Phase 3 Clinical | Cspc Baike (Shandong) Biopharmaceutical Co Ltd, Synermore Biologics (Suzhou) Co Ltd | Chronic Urticaria | Details |
| Omalizumab biosimilar (Reliance Life Sciences) | R-TPR-022 | Phase 3 Clinical | Reliance Life Sciences | Asthma | Details |
| Omalizumab biosimilar (Teva) | TEV-45779 | Phase 3 Clinical | Teva Pharmaceutical Industries Ltd | Chronic Urticaria | Details |
| Ligelizumab | QGE-031 | Phase 3 Clinical | Genentech Inc | Chronic Urticaria; Food Hypersensitivity; Pemphigoid, Bullous; Asthma; Urticaria; Peanut Hypersensitivity; Dermatitis, Atopic; Hypersensitivity | Details |
| Omalizumab biosimilar (BiosanaPharma) | ADL-018; AVT-23; BP-001 | Phase 3 Clinical | BiosanaPharma BV | Chronic Urticaria; Allergic asthma | Details |
| Recombinant anti-IgE monoclonal antibody(Cspc Baike) | Phase 3 Clinical | Cspc Baike (Shandong) Biopharmaceutical Co Ltd | Chronic Urticaria; Asthma | Details | |
| Omalizumab biosimilar (Yuanda Shuyang) | SYB-507 | Phase 3 Clinical | Sichuan Yuanda Shuyang Pharmaceutical Co Ltd | Chronic Urticaria; Allergic asthma; Asthma | Details |
| Anti-CEmX monoclonal antibody (Fountain Biopharma) | h4B12; FB-825 | Phase 2 Clinical | Academia Sinica | Asthma; Dermatitis, Atopic | Details |
| UB-221 | UB-221 | Phase 2 Clinical | Chinese Academy Of Sciences | Chronic Urticaria | Details |
| Omalizumab biosimilar (Glenmark) | GBR-310 | Phase 2 Clinical | Glenmark Pharmaceuticals Ltd | Chronic Urticaria; Asthma | Details |
| Bifidobacterium Bifidum(Sun Yat-sen University) | Phase 2 Clinical | Sun Yat-Sen University | Carcinoma, Hepatocellular | Details | |
| LP-003 (Tianchen Biomedical) | LP-003 | Phase 2 Clinical | Longbio Pharma (Suzhou) Co Ltd | Rhinitis, Allergic, Seasonal; Chronic Urticaria; Nasal Polyps; Rhinitis, Allergic; Allergic asthma; Asthma; Sinusitis | Details |
| ADP-101 | ADP-101 | Phase 2 Clinical | Alladapt Immunotherapeutics Inc | Food Hypersensitivity | Details |
| Omalizumab biosimilar (Fountain BioPharma) | FB-317 | Phase 1 Clinical | Academia Sinica | Asthma; Urticaria | Details |
| Omalizumab biosimilar(Yuhan) | YH-35324 | Phase 1 Clinical | Yuhan Corp | Chronic Urticaria; Immune System Diseases; Urticaria; Hypersensitivity | Details |
| IGNX-001 | IGNX-001; IGNX001; IGX-0107 and IGX-0109 | Phase 1 Clinical | IgGenix Inc | Peanut Hypersensitivity | Details |
| JYB-1904 | JYB-1904 | Phase 1 Clinical | Jiangsu Jiye Biopharmaceutical Co Ltd | Allergic asthma; Urticaria; Asthma | Details |
| Omalizumab biosimilar (Tianchen Biomedical) | Phase 1 Clinical | Longbio Pharma (Suzhou) Co Ltd | Chronic Urticaria | Details | |
| Anti-IgE allergy vaccine (United Biomedical) | Phase 1 Clinical | United Biomedical Inc | Hypersensitivity | Details | |
| Omalizumab biosimilar (Synermore Biologics) | SYN-008 | Phase 3 Clinical | Cspc Baike (Shandong) Biopharmaceutical Co Ltd, Synermore Biologics (Suzhou) Co Ltd | Chronic Urticaria | Details |
| Omalizumab biosimilar (Reliance Life Sciences) | R-TPR-022 | Phase 3 Clinical | Reliance Life Sciences | Asthma | Details |
| Omalizumab biosimilar (Teva) | TEV-45779 | Phase 3 Clinical | Teva Pharmaceutical Industries Ltd | Chronic Urticaria | Details |
| Ligelizumab | QGE-031 | Phase 3 Clinical | Genentech Inc | Chronic Urticaria; Food Hypersensitivity; Pemphigoid, Bullous; Asthma; Urticaria; Peanut Hypersensitivity; Dermatitis, Atopic; Hypersensitivity | Details |
| Omalizumab biosimilar (BiosanaPharma) | ADL-018; AVT-23; BP-001 | Phase 3 Clinical | BiosanaPharma BV | Chronic Urticaria; Allergic asthma | Details |
| Recombinant anti-IgE monoclonal antibody(Cspc Baike) | Phase 3 Clinical | Cspc Baike (Shandong) Biopharmaceutical Co Ltd | Chronic Urticaria; Asthma | Details | |
| Omalizumab biosimilar (Yuanda Shuyang) | SYB-507 | Phase 3 Clinical | Sichuan Yuanda Shuyang Pharmaceutical Co Ltd | Chronic Urticaria; Allergic asthma; Asthma | Details |
| Anti-CEmX monoclonal antibody (Fountain Biopharma) | h4B12; FB-825 | Phase 2 Clinical | Academia Sinica | Asthma; Dermatitis, Atopic | Details |
| UB-221 | UB-221 | Phase 2 Clinical | Chinese Academy Of Sciences | Chronic Urticaria | Details |
| Omalizumab biosimilar (Glenmark) | GBR-310 | Phase 2 Clinical | Glenmark Pharmaceuticals Ltd | Chronic Urticaria; Asthma | Details |
| Bifidobacterium Bifidum(Sun Yat-sen University) | Phase 2 Clinical | Sun Yat-Sen University | Carcinoma, Hepatocellular | Details | |
| LP-003 (Tianchen Biomedical) | LP-003 | Phase 2 Clinical | Longbio Pharma (Suzhou) Co Ltd | Rhinitis, Allergic, Seasonal; Chronic Urticaria; Nasal Polyps; Rhinitis, Allergic; Allergic asthma; Asthma; Sinusitis | Details |
| ADP-101 | ADP-101 | Phase 2 Clinical | Alladapt Immunotherapeutics Inc | Food Hypersensitivity | Details |
| Omalizumab biosimilar (Fountain BioPharma) | FB-317 | Phase 1 Clinical | Academia Sinica | Asthma; Urticaria | Details |
| Omalizumab biosimilar(Yuhan) | YH-35324 | Phase 1 Clinical | Yuhan Corp | Chronic Urticaria; Immune System Diseases; Urticaria; Hypersensitivity | Details |
| IGNX-001 | IGNX-001; IGNX001; IGX-0107 and IGX-0109 | Phase 1 Clinical | IgGenix Inc | Peanut Hypersensitivity | Details |
| JYB-1904 | JYB-1904 | Phase 1 Clinical | Jiangsu Jiye Biopharmaceutical Co Ltd | Allergic asthma; Urticaria; Asthma | Details |
| Omalizumab biosimilar (Tianchen Biomedical) | Phase 1 Clinical | Longbio Pharma (Suzhou) Co Ltd | Chronic Urticaria | Details | |
| Anti-IgE allergy vaccine (United Biomedical) | Phase 1 Clinical | United Biomedical Inc | Hypersensitivity | Details |
This web search service is supported by Google Inc.